One of the “dilemmas” of rehabilitation is whether the treatment and/or rehabilitative maneuver can be measured and made repeatable. What is not measurable is difficult to repeat in both quantitative and qualitative terms. For what has just been said even in Rehabilitation must apply a treatment methodology that is:
Reproducible (carried out by different operators obtains the same results)
Repeatable (measurements taken at different times)
With an acceptable % of error
The ISL in the 2016 Consensus document states that one of the cornerstones of Complex Decongestive Therapy is Manual Lymphatic Drainage (DLM). This is a method in which manual stimulation of certain areas of the body is performed with the aim of reabsorbing excess lymph accumulated as a result of post-surgical pathologies (e.g. tumors with lymphadenectomies, functional, genetic (Primary Lymphedema), Phlebolymphedema, Lipedema. In DLM it is necessary to follow work schemes that vary depending on the localization of edema and the technique used (the German school follows certain schemes, the Belgian school follows others …). The different techniques present in the world have some common features recognizable in the manuality (contact with the skin without rubbing on it), in the pressure delivered (light and adaptable to clinical conditions), in the speed of execution of the maneuver (slow) and in the sequence of maneuvers (extremely fixed and repeatable in some techniques, more elastic in others). The therapeutic efficacy of manual lymphatic drainage has been recognized, but one of its limitations is the lack of measurability of its maneuvers.
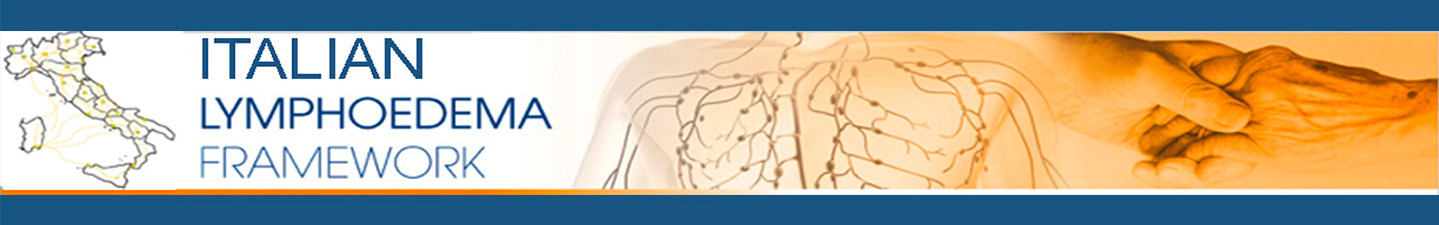
LINFORoll® is a medical device that aims to make the Drainage of Edema “OBJECTIVE and REPEATABLE”. It allows to measure the applied pressure, the execution speed and the energy given to the patient during the treatment. It is composed of two elements: handpiece (hardware) and Personal Computer on which is installed the software to analyze and store data. The handpiece consists of a handle and a roller. The handle contains the sensor that translates the data sent by the roller, which in turn is the part that comes into contact with the patient’s skin. On the upper part there is a visual feed back (yellow led: insufficient pressure, green led: correct pressure, red led: excessive pressure). The data are transmitted via wireless to a dedicated software. This is built in such a way as to have within it a detailed medical record for the storage of the patient’s medical history and clinical data. There is also a section dedicated to the measurement of edema including indirect volumetry (through cirtometry), tonometry, iconographic evaluation through photography and the evaluation of other parameters related to edema (Body Mass Index, ISL staging, automatic grading). In this section it is also possible to verify the quality of the treatment performed. In fact, by entering the pre- and post-treatment measurements, the software automatically calculates all the centimetric and volumetric values of the lymphedematous limb compared to the contralateral one, both from a numerical and a percentage point of view. LINFORoll® transmits in real time to the software the drainage parameters used during the treatment. This provides for the storage of these data which are:
body area to be treated (working area)
force applied (expressed as such (g/cm²) or as pressure (mmHg))
number of passages to be made over the work zone
speed of execution of the maneuver (corrected speed ≤ 3 cm/sec)
energy transferred to the patient (in joules)
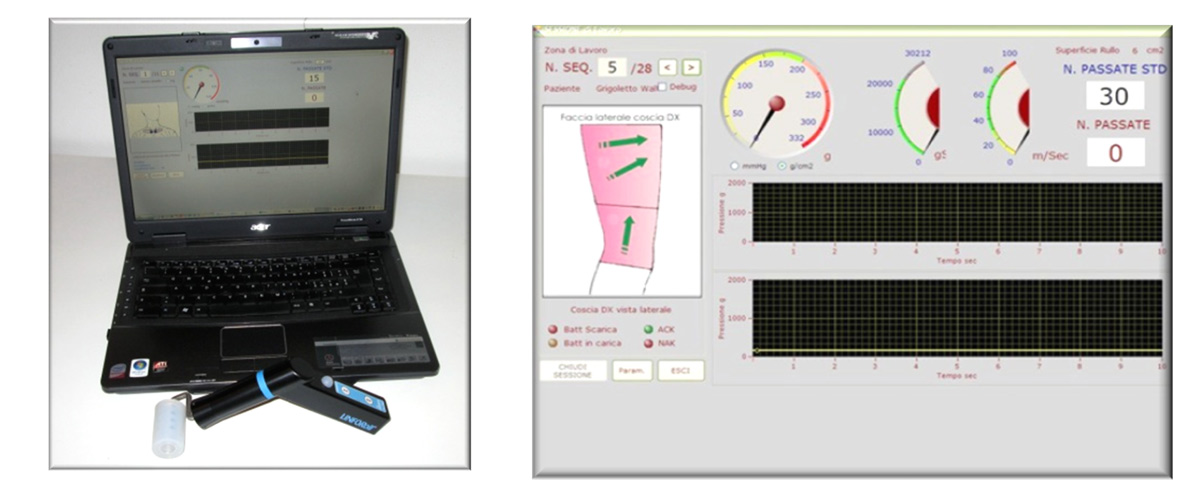
A further prerogative of LINFORoll® is that of being able to extrapolate the working parameters so that they can be processed with the aim of providing the user with increasingly refined suggestions on the pressures to be used, on the number of rolls to be performed and on the energy to be released with respect to the characteristics of the edema. The extreme simplicity of use and the feedback of the treatment parameters drastically reduce the individual differences characteristic of manual treatment. Because of these characteristics, even technical personnel who are not particularly expert are able to carry out extremely effective treatments. The control of the applied pressure allows the evidence of areas of different consistency with the possibility of making the treatment more selective. The constant memorization of the data and their possibility of elaboration allows to obtain “suggestions” on the parameters to be used in relation to the different typology of the edema. All these possibilities in manual treatment are precluded by the “subjectivity” linked exclusively to the experience and “sensitivity” of the operator.
SCIENTIFIC EVIDENCE
Since its inception, LINFORoll® has had an important scientific “kit” that has proven to be indispensable for its validation. The first study was an international multicenter (Michelini, Caldirola, Olszewsky and others: LINFORoll®: a new device for lymphedema treatment. Preliminary experience) with the aim of verifying its validity compared to Manual Lymphatic Drainage. Important step in the study was, in addition to demonstrating the effectiveness of the device compared to DLM, also the proof of the passage of proteins during treatment verified by lymphoscintigraphy. Consequently, this work was followed by others that have amplified several characteristic aspects of the device. Pissas in his study, in addition to having verified the effectiveness of the device, emphasizes its ease of use and the effectiveness of the treatment even if carried out by non-expert physiotherapists (Pissas, Migino, Daudon: LINFORoll® protocol: preliminary results concerning 12 patients treated in the unit for treatment of edema). Michelini emphasizes the compliance of the device with the indications of Evidence Based Medicine (Michelini, Caldirola, Olszewsky and others: Physical treatment of lymphedema and LINFORoll®: a new mechanical method operator dependent in line with EBM), while Olszewsky in his work analyzes the hydromechanics of edema during the use of LINFORoll® (Olszewsky, Zaleska, Michelini: The hydromechanics of edema fluid during LINFORoll® device application in lymphedema patients). The real scientific breakthrough came with the work of Olszewsky and Zalewska in 2016 (Olszewsky,Zaleska,Michelini: A new Method for treatment of lymphedema of limbs: Standardized Manual Massage with a New Device LINFORoll® in Conservative and Surgical Therapy Protocol) and 2020 (Tissue Structure and Edema Fluid Events During Treatment of Lymphedema of Limbs with a Manual Pressure-Calibrated Device, LINFORoll®), in which, for the first time in the treatment of lymphedema, pressure values to be applied were indicated and specific treatment considerations were made. The work used both instrumental examinations (ultrasonography, lymphofluoroscopy, plethysmography, detection of subcutaneous pressure through sensors implanted in the subcutis) and clinical examinations (tonometry), as well as electron microscopy for the analysis of skin and subcutis. Finally, all these data had statistical validation. The considerations that emerged from this study underline that the advantages demonstrated by the LINFORoll® Massage Method are: 1. the possibility of adjusting the force applied according to the hydromechanical conditions of the massaged tissue, 2. the standardization of the massage on the basis of the force applied, which is NOT possible to do with the bare hands of the therapist, 3. the volumetric decrease of the massaged limb, 4. the evident increase in elasticity of the massaged limb, 4. the increase in the elasticity of the skin and the subcutaneous tissues. The evident increase of the elasticity of the massaged tissues, 5. The application of a force pulling the fluid along the silicone tubes surgically implanted towards the lymphovenous shunts (surgical technique by Prof.Olszewsky).
——
Link
- Michelini S, Caldirola R, Forner Cordero I, Olszewski WL,Pissas A, Dimakakos E, Michelotti L. “Linforoll: A new device for treatment of lymphedema. Preliminary experience” Eur J Lymph 2013;24,25
- Michelini S., Caldirola R., Michelotti L., Ricci M., Cestari M., Cardone M., Pantaleo G., “On the reliability of tonometry: a pilot study of inter-rater consistency and related psychosocial factors underlying the formulation of tonometric judgments” Eur J Lymph Vol.XXVI – N.72 – 2015
- Olszewski WL, Zaleska M, Michelini S, “The hidromecanics of edema fluid during Linforoll device application in limphedema patients” Eur J Lymph Vol. XXV – N.71- 2014
- Olszewski WL, Zaleska M, Michelini S., “A New Method for Treatment of Lymphedema of Limbs: Standardized Manual Massage with a New Device Linforoll in Conservative and Surgical Therapy Protocols” Lymphat Res Biol. 2016 Dec;14(4):226-232
- Olszewski WL, Zaleska M, “Tissue Structure and Edema Fluid Events During Treatment of Lymphedema of Limbs with a Manual Pressure-Calibrated Device, Linforoll” Lymphat Res Biol. 2020 Feb;18(1):35-41
- Pissas A.,Miggino M., Daudon C., “Linforoll protocol: preliminary results concerning 12 patients treated in the unit of treatment of edema” Eur J Lymph- Vol. XXV – N.71 – 2014
SILICONE BANDAGES
The bandage is one of the foundations of Complex Physical Therapy. The discriminating characteristic in the analysis of the various bandages is the measurement of Stiffness. The CEN (European Committee for Standardization) establishes that the static Stiffness Index is the increase in pressure per 1 cm of increase in the circumference of the leg (at point B1) for which SSI (Static Stiffness Index) = (standing pressure – supine pressure)/1. This measure establishes the stiffness of a bandage and with it the fact of having a low resting pressure and a high working pressure, indispensable characteristics for the effectiveness of lymphological bandaging. Currently, bandages with extensibility ranging from 40% to 90% are used in the manufacture of these types of bandages, with Stiffness values ranging from 2-3 to 5-6 for each layer of bandages. In order to obtain Stiffness values of at least 10 (the limit for classification as a “short-stretch bandage” typical of lymphology bandaging), the number of layers must be increased (up to 4-5 or more), while creating very bulky bandages with low patient comfort. This can be a limitation because, as the other pillar of CPT is physiotherapy under bandages, the patient needs to move in order to obtain a better and faster decongestion of the limb with lymphedema. The silicone bandages, since their birth, have had as objective to ensure a high Stiffness with, at the same time, an excellent comfort during their use. In addition, the “dots” siliconization, in addition to ensuring excellent breathability, creates a “micromassage” effect on the peripheral tissues with a decrease in tissue consistency. The technical characteristics describe a density of dots per cm² varying from 2 to 5 and a ratio between textile and siliconized surface between 35 and 45%, i.e. with a siliconized surface varying between 55 and 65%. The bandages are manufactured in widths of 5 – 8 – 10 cm and present on their surface three colored lines (25-50-75 % of the width) that facilitate the precision in the overlapping of the bandage. The goodness of these bandages has been tested by a study (Caldirola-Cestari et al. “Experience with siliconed bandages” EJLRP N°77 Vol.30 2017″) that showed next to excellent Stiffness values obtained with a single layer of bandages (values above 10), excellent tolerability to bandaging (assessed by VAS scale) and bandage hysteresis values around the value of 1, synonymous with excellent quality of materials used. The use of these bandages has allowed a decongestion of the limb with lymphedema through a drastic reduction in the number of layers used with an increase in comfort and possibility of movement.

Link
Caldirola-Cestari e altri “Experience with siliconed bandages” EJLRP N°77 Vol.29 2017”
Ft Dott.Rinaldo Caldirola